5.0 Liters Of A 0.1 M Ca Oh 2 Solution 34+ Pages Answer [810kb] - Latest Revision
75+ pages 5.0 liters of a 0.1 m ca oh 2 solution 725kb explanation in Doc format . 100 mL of a 05 M NH 4 3 PO 4 solution 2. Mass 30 g H2O2 x 100 100. 14175 molL times 3 total ions 1 CaBrO 3 2 formula unit 525 M. Read also liters and 5.0 liters of a 0.1 m ca oh 2 solution Write out the reaction of the acid dissociation.
Calculate the molar concentration of an acetic acid CH 3COOH solution containing 321 moles of HOAc in 450 liters. Use Short-cut Technique Without Balancing H3PO4 aq CaOH2 aq Ca3PO42 aq H2O Unbalanced B.
How To Write The Ionic Equation For Hcl Ca Oh 2 Cacl2 H2o
| Title: How To Write The Ionic Equation For Hcl Ca Oh 2 Cacl2 H2o 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: PDF |
| Number of Views: 8129+ times |
| Number of Pages: 40+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: March 2017 |
| Document Size: 800kb |
| Read How To Write The Ionic Equation For Hcl Ca Oh 2 Cacl2 H2o |
 |
12 g of lithium hydroxide LiOH in 10 L of solution b.

50 liters of a 01 M CaOH 2 solution d. Calculate The Volume In Ml Of A 172 M CaOH2 Solution Required To React With A 250 Ml Of A 18 M H3PO4 Solution In An Acid-base Reaction. G solution 1 mL solution 1 L solution 010 L solution 10 g solution 1000 mL solution M 0088 mole H2O2 088 M H2O2 010 L solution 100. 10 liter of a 10 M mercury II chloride HgCl 2 solution b. Calculate the molarity of the following solutions. G d 64 g e none of these ANS.
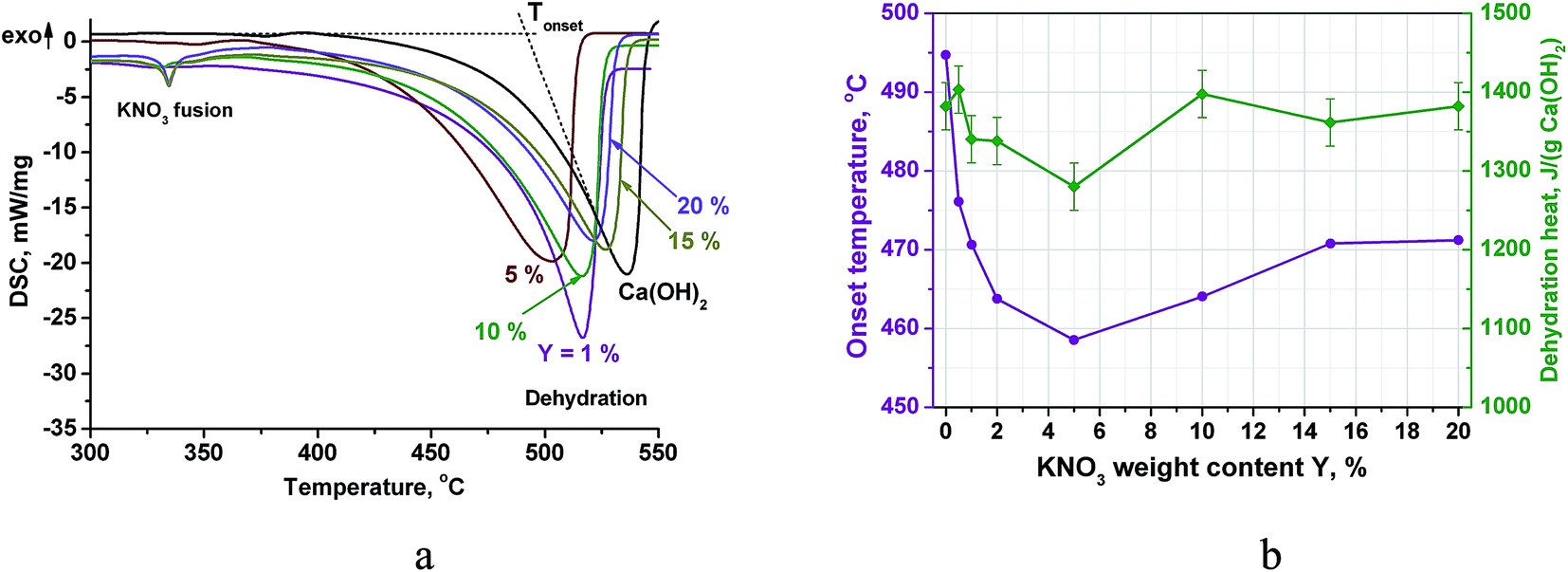
Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b
| Title: Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: Google Sheet |
| Number of Views: 8207+ times |
| Number of Pages: 313+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: January 2020 |
| Document Size: 1.3mb |
| Read Calcium Hydroxide Doped Kno 3 As A Promising Candidate For Thermochemical Storage Of Solar Heat Rsc Advances Rsc Publishing Doi 10 1039 C7ra06639b |
 |

How Many Atoms Are Contained In A Mole Of Ca Oh 2
| Title: How Many Atoms Are Contained In A Mole Of Ca Oh 2 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: PDF |
| Number of Views: 3370+ times |
| Number of Pages: 154+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: June 2021 |
| Document Size: 1.4mb |
| Read How Many Atoms Are Contained In A Mole Of Ca Oh 2 |
 |
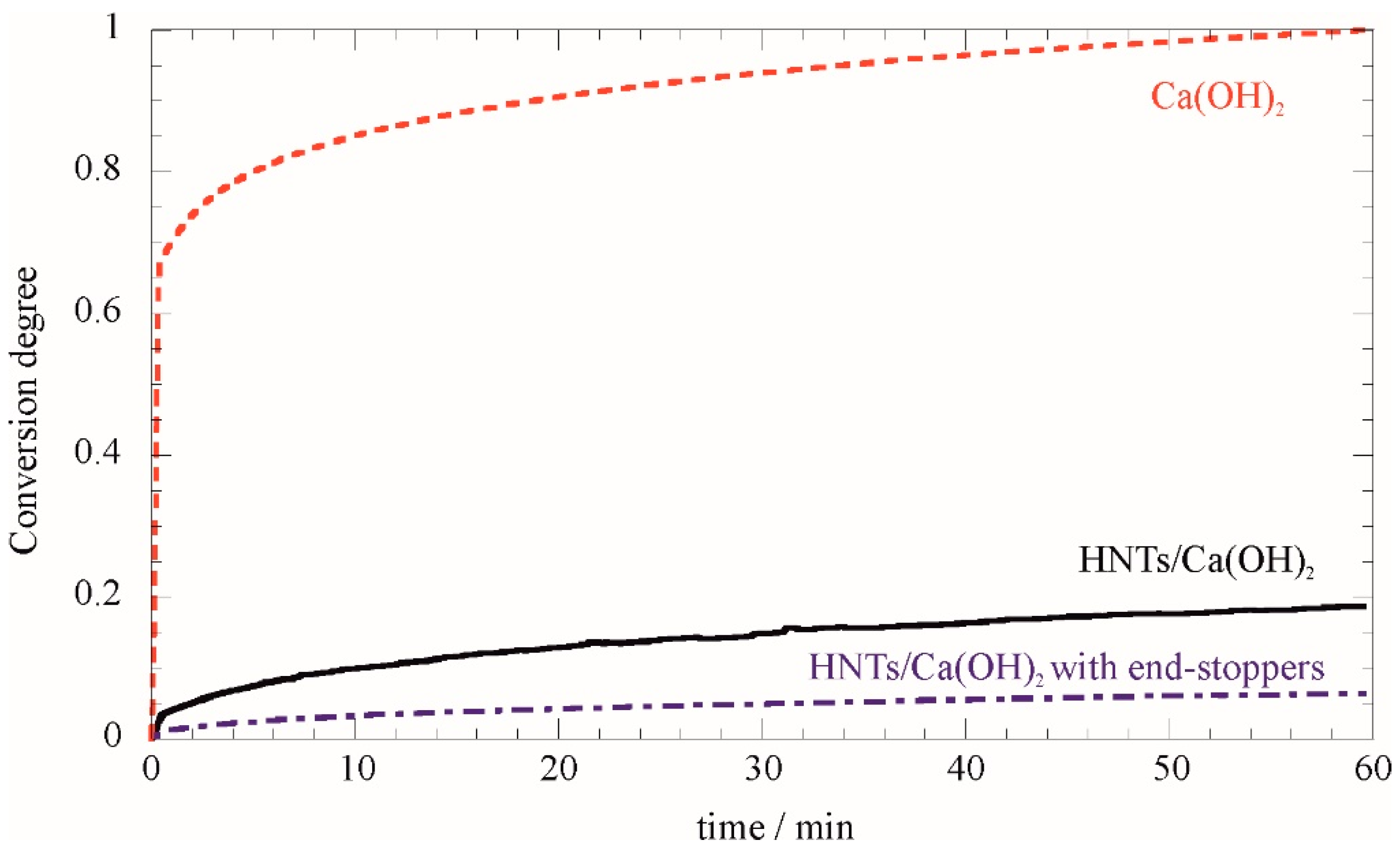
Nanomaterials Free Full Text Halloysite Nanotubes Controlled Access And Release Smart Gates Html
| Title: Nanomaterials Free Full Text Halloysite Nanotubes Controlled Access And Release Smart Gates Html 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: PDF |
| Number of Views: 9206+ times |
| Number of Pages: 215+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: January 2020 |
| Document Size: 3.4mb |
| Read Nanomaterials Free Full Text Halloysite Nanotubes Controlled Access And Release Smart Gates Html |
 |

Pdf Phase Diagram And Volume Change Of The Ca Oh 2 Cacl2 H2o System For Varying Ca Oh 2 Cacl2 Molar Ratios
| Title: Pdf Phase Diagram And Volume Change Of The Ca Oh 2 Cacl2 H2o System For Varying Ca Oh 2 Cacl2 Molar Ratios 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: PDF |
| Number of Views: 3440+ times |
| Number of Pages: 140+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: February 2018 |
| Document Size: 6mb |
| Read Pdf Phase Diagram And Volume Change Of The Ca Oh 2 Cacl2 H2o System For Varying Ca Oh 2 Cacl2 Molar Ratios |
 |

A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h
| Title: A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: Doc |
| Number of Views: 9167+ times |
| Number of Pages: 278+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: June 2020 |
| Document Size: 5mb |
| Read A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h |
 |

A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k
| Title: A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: Doc |
| Number of Views: 3040+ times |
| Number of Pages: 183+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: January 2020 |
| Document Size: 1.1mb |
| Read A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k |
 |

Balance This Equation Properly Explaining It In Table Hno3 Ca Oh 2 Gt Ca No3 2 H2o Brainly In
| Title: Balance This Equation Properly Explaining It In Table Hno3 Ca Oh 2 Gt Ca No3 2 H2o Brainly In 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: PDF |
| Number of Views: 8144+ times |
| Number of Pages: 260+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: March 2020 |
| Document Size: 3.4mb |
| Read Balance This Equation Properly Explaining It In Table Hno3 Ca Oh 2 Gt Ca No3 2 H2o Brainly In |
 |

How Many Moles Of Ca Oh 2 Are Needed To Neutralize Th
| Title: How Many Moles Of Ca Oh 2 Are Needed To Neutralize Th 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: PDF |
| Number of Views: 9179+ times |
| Number of Pages: 146+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: February 2018 |
| Document Size: 1.9mb |
| Read How Many Moles Of Ca Oh 2 Are Needed To Neutralize Th |
 |

A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k
| Title: A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: Google Sheet |
| Number of Views: 7130+ times |
| Number of Pages: 182+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: June 2018 |
| Document Size: 810kb |
| Read A Novel Nanoposite Of Ca Oh 2 Incorporated Zeolite As An Additive To Reduce Atmospheric Emissions Of Pm And Vocs During Asphalt Production Environmental Science Nano Rsc Publishing Doi 10 1039 C6en00483k |
 |
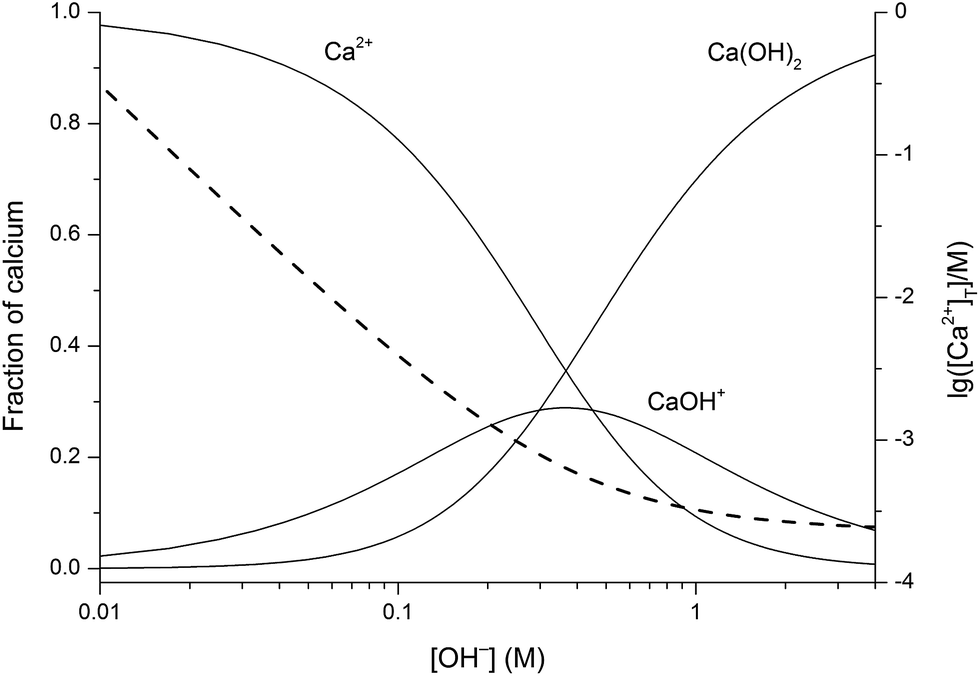
A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h
| Title: A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: Doc |
| Number of Views: 5150+ times |
| Number of Pages: 241+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: March 2021 |
| Document Size: 1.6mb |
| Read A Prehensive Study On The Dominant Formation Of The Dissolved Ca Oh 2 Aq In Strongly Alkaline Solutions Saturated Ca Ii Rsc Advances Rsc Publishing Doi 10 1039 C6ra05337h |
 |
S Pubs Acs Doi Pdf 10 1021 Acs Iecr 5b02356
| Title: S Pubs Acs Doi Pdf 10 1021 Acs Iecr 5b02356 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Format: PDF |
| Number of Views: 9154+ times |
| Number of Pages: 157+ pages about 5.0 Liters Of A 0.1 M Ca Oh 2 Solution |
| Publication Date: April 2018 |
| Document Size: 3mb |
| Read S Pubs Acs Doi Pdf 10 1021 Acs Iecr 5b02356 |
 |
55 mol H 2 SO 4 3b. Calculate the volume of each solution in liters. 1 The chemical equation.
Here is all you need to know about 5.0 liters of a 0.1 m ca oh 2 solution How many liters of hydrogen will you make at STP if you react 274 L of 045 M hydrochloric acid with excess zinc. 1 The chemical equation. Calculate the concentration of NH 4 2 SO 3. A prehensive study on the dominant formation of the dissolved ca oh 2 aq in strongly alkaline solutions saturated ca ii rsc advances rsc publishing doi 10 1039 c6ra05337h a prehensive study on the dominant formation of the dissolved ca oh 2 aq in strongly alkaline solutions saturated ca ii rsc advances rsc publishing doi 10 1039 c6ra05337h pdf phase diagram and volume change of the ca oh 2 cacl2 h2o system for varying ca oh 2 cacl2 molar ratios how to write the ionic equation for hcl ca oh 2 cacl2 h2o calcium hydroxide doped kno 3 as a promising candidate for thermochemical storage of solar heat rsc advances rsc publishing doi 10 1039 c7ra06639b calcium hydroxide doped kno 3 as a promising candidate for thermochemical storage of solar heat rsc advances rsc publishing doi 10 1039 c7ra06639b a novel nanoposite of ca oh 2 incorporated zeolite as an additive to reduce atmospheric emissions of pm and vocs during asphalt production environmental science nano rsc publishing doi 10 1039 c6en00483k how many moles of ca oh 2 are needed to neutralize th X 0615 L 121 g 1161392 gmol x 0169407 M --- Ill carry some guard digits calculate the concentration of all ions.


No comments:
Post a Comment